Research
Research Activity
During the course of our work at Tel-Aviv University, we developed novel molecular systems with self-immolative capabilities. The ability of a molecule to undergo spontaneuse domino-like disassembly upon a stimulus event is refered as self-immolative feature. Intially, we described a chemical adaptor system for targeted prodrug approach. The prodrug is disaasembled of the targeting moiety to realse the active drug upon a triggering event. Next, we develoed dendrimers with self-immolative capabilities and recently, we extanded this approach to linear polymers and comb polymers.
Chemical Adaptor Systems
We have developed a drug delivery system based on a chemical adaptor that provides a generic linkage of a drug with a targeting device in a manner set to be triggered by defined enzymatic activity (Figure 1). The system is generic and allows using a variety of drugs, targeting devices, and enzymes by introducing the corresponding substrate as a trigger for drug release in the chemical adaptor. The chemical adaptor system was designed with stable chemical linkages, in order to avoid nonspecific drug release in vivo. Proof of concept was demonstrated using etoposide as the drug, an HPMA-copolymer as the targeting device and catalytic antibody 38C2 as the triggering enzyme (Figure 2).
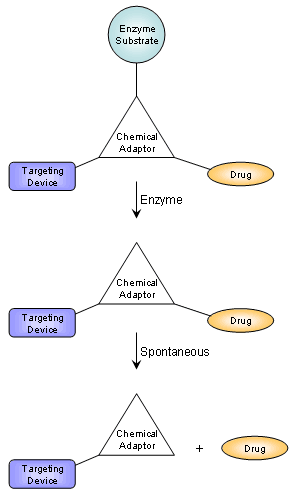
Figure 1: General design of the chemical adaptor system. Cleavage of the enzyme substrate generates an intermediate that spontaneously rearranges to release the drug from the targeting device.

Figure 2: Mechanism of etoposide drug release from the HPMA-copolymer, using catalytic antibody 38C2 as the triggering enzyme.
Self-Immolative Dendrimers
Self-immolative dendrimers are unique structural molecules that can release all of their tail units, through a domino-like chain fragmentation, which is initiated by a single cleavage at the dendrimer's core (Figure 3). Incorporation of drug molecules as the tail units and an enzyme substrate as the trigger, can generate a multi-prodrug unit that is activated with a single enzymatic cleavage. Dendritic prodrugs, activated through a single catalytic reaction by a specific enzyme, were shown to present significant advantages in the inhibition of tumor growth, especially if the targeted or secreted enzyme exists at relatively low levels in the malignant tissue. Self-immolative dendrimers were also applied as a general platform for biosensor molecules, which are used to detect/amplify enzymatic activity. The development and evaluation of self-immolative dendrimers so far, generate seventeen publications. The concept was also highlighted in Nature and in Chemical & Engeenering News.

Figure 3: Self-immolative dendrimers, as shown in the picture, spontaneously release all the end-group molecules following a single activation event. This triggering event induces a cascade of self-eliminations, which leads to complete dissociation of the dendrimer into its separate building blocks.
Self-Immolative Polymers
Smart polymers are special kinds of polymeric molecules that respond to external stimuli. We have developed a novel smart polymer designed to sequentially disassemble into its building blocks upon initiation by a triggering event at the polymer head (Figure 4). The polymer structure is based on a polycarbamate backbone that disassembles through a domino-like, 1,6-elimination and decarboxylation reactions. To demonstrate the concept, we synthesized a self-immolative polymer that amplifies a single cleavage reaction into multiple-release of fluorogenic molecules and confirmed the head to tail disassembly concept. These polymers can be used to prepare highly sensitive molecular sensors with large signal-to-noise ratios. The sensors should be useful for the detection of a wide range of biological and chemical activities through use of the appropriate trigger at the polymer-head.
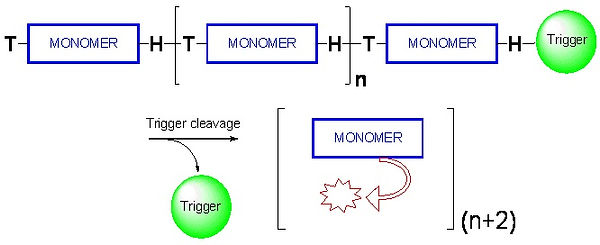
Figure 4: Illustration of the disassembly of a self-immolative polymer.
Insertion of additional substituents at a specific position of each monomer generates self-immolative comb-polymers with side-releasable groups that could be used as a drug delivery system (Figure 5). Upon activation of the head group, the comb-polymer undergoes complete disassembly to release multiple copies of side-reporter group. The polymer was prepared by simple polymerization of a monomer, followed by capping of the polymer head with a trigger. This technique allowed rapid synthesis of the polymeric molecule containing a large number of reporter units. We demonstrate that a water-soluble version of the comb-polymer can be activated by an enzyme and thus has potential as selctive drug delivery system.

Figure 5: Illustration of the disassembly of a self-immolative comb-polymer.
Chemotherapeutic Bone-Targeted Bisphosphonate Prodrugs with Hydrolytic Mode of Activation
Our group is also deveoping prodrugs for selective chemotherapy a representetive example is described below:
Osseous tissues are considered to be limited as therapeutic target sites due to their biological properties. We have designed and synthesized two kinds of hydrolytically-activated chemotherapeutic prodrugs containing bisphosphonate, a bone-targeting moiety. The first can be conjugated to drug molecules with an available hydroxy group; the drug is attached to the bisphosphonate component through an ester-labile linkage. The second is for use with drug molecules with amine functional group. In this case, a self-immolative linker is used to attach the drug to the bisphosphonate component through a carbonate-labile linkage. The concept was demonstrated using the drugs camptothecin, which has a hydroxy functional group, and tryptophan, which is a model molecule for a drug with amine functionality. Both prodrugs showed significant binding capability to hydroxyapatite, the major component of bone, and were hydrolytically activated under physiological conditions.

Figure 6: Camdronate - A chemotherapeutic Bone-Targeted Bisphosphonate Prodrug with Hydrolytic Mode of Activation
Enhanced Chemiluminescence via Structural Innovation in Dioxetane Probes
Chemiluminescent probes are powerful diagnostic tools offering high sensitivity and signal-to-noise ratios for biological detection and imaging. Over the last few years, we developed a dual-strategy advancement in dioxetane-based probe design that integrates electronic substitution and mechanical strain modulation to achieve unprecedented performance in aqueous chemiluminescence. First, we employed ortho-substituted electron-withdrawing groups (EWGs), such as methyl acrylate and acrylonitrile, to significantly enhance fluorescence and chemiluminescence quantum yields—achieving values up to 9.8% under physiological conditions, representing a >3000-fold improvement over traditional adamantylidene-dioxetanes.

Figure 7: Opening a Gateway for Chemiluminescence Cell Imaging: Distinctive Methodology for Design of Bright Chemiluminescent Dioxetane Probes
Second, we introduce a novel spirostrain release approach by replacing the adamantyl scaffold with spiro-cyclobutyl or spiro-oxetanyl units. This architectural change accelerates the chemiexcitation rate by 107- to 2662-fold, enabling rapid light emission in a flash-type kinetic mode while maintaining high stability and brightness.

Figure 8: Spirostrain-Accelerated Chemiexcitation of Dioxetanes Yields Unprecedented Detection Sensitivity in Chemiluminescence Bioassays
Combining these design principles, we developed single-component, aqueous-compatible probes capable of imaging endogenous β-galactosidase activity in live cells—the first such demonstration using a non-luciferin chemiluminescent mechanism. Moreover, a methyl acrylate cyclobutyl-dioxetane probe detected Escherichia coli with a limit of detection of 2,560 cells, a 125-fold sensitivity enhancement over the corresponding adamantyl analogue, and exhibited a signal-to-noise ratio exceeding 400,000.
These results define a new class of structurally optimized chemiluminescent luminophores with exceptional emissive efficiency, kinetic performance, and practical utility in biological assays. The modular design further enables facile adaptation to diverse enzymatic and analyte-responsive triggers, positioning these probes as state-of-the-art tools for next-generation bioimaging and diagnostic applications.

