Publications
Lu, Y., Huang, K.B., Shabat, D.
Sunlight-powered photodynamic therapy for painless diabetic wound care
National. Sci. Rev., 2026, doi.org/10.1093/nsr/nwag033.
169.
168.
Scott, J.I., Cheng, Z., Thompson, E.J., Karmakar, U., Cowell, V., David, M., Gordon, D., Mendive-Tapia, L., Le Saint-Grant, A., Volkmer, P., Chuah, C.S., Lau, P., Rossi, A.G., Nagengast, W.B., Shabat, D., Ho, G.T., Vendrell, M.
An activity-based chemiluminescence assay targeting granzyme A for monitoring Inflammatory Bowel Diseases
Nat. Biomed. Eng., 2026, doi.org/10.1038/s41551-025-01588-1

167.
Zhu, J., Gutkin, S., Chen, S., Su, SP., Phan, H., Shabat, D., Bogyo, M.
Chemiluminescent probes for imaging cysteine cathepsin activity
Bio. Org. Med. Chem. Lett., 2025, https://doi.org/10.1016/j.bmcl.2025.130480

166.
David, M., Leirikh, T., Naama, N., Kopp, T., Shabat, D.
Phenylamine-1,2-Dioxetanes: Promising Class of Chemiluminescent Luminophores for Aqueous Sensing and Bacterial Detection
Angew. Chem. Int. Ed., 2025, 64, e202515674

165.
Kaufman, F., David, M., Zaiden, M., Shabat, D., Amiram, M.
Imparting New Stimuli-Responsive Behaviors in Protein-polymers via Self-Immolative Linker Conjugation
Chem. B. 2025, 13, 12276-292.
164.
Tannous, R., Zhang, C., Shabat, D.
Super Sensitive Chemiluminescent Probe for Detection of Caspase-3 Activity
Bioconjug. Chem., 2025, 2025, 36, 1113−1120

163.
Tannous, R., Shabat, D.
Structure-Activity Optimization of Phenoxy-1,2-Dioxetane Precursors as Probes for Singlet Oxygen Yields Unprecedented Detection Sensitivity
JACS Au, 2025, 5, 2871−2883

162.
Shelef, O., Gutkin, S., Nassir, M., Krinsky, A., Satchi-Fainaro, R., Baran, P.S., Shabat, D.
Thymidine Phosphodiester Chemiluminescent Probe for Sensitive and Selective Detection of Ectonucleotide Pyrophosphatase 1
Bioconjug. Chem., 2025, 36, 152-159

161.
David, M., Gutkin, S., Nithun, R.V., Jbara, M., Shabat.
Unprecedented Photoinduced-Electron-Transfer Probe with a Turn-ON Chemiluminescence Mode-of-Action

160.
Shelef, O., Krinsky, A., Jospe-Kaufman, M., Babjaková, Z., Fridman, M., Satchi-Fainaro, R., Spitz, S., Shabat., D.
Biocompatible Flash Chemiluminescent Assay Enabled by Sterically Hindered Spiro-Strained-Oxetanyl-1,2-Dioxetane
Chem. Eur. J., 2024, e202402981

159.
Gutkin, S., Shelef, O., Babjaková, Z., Tomanová, L.A., Babjak, M., Spitz, U., Zhou, Q., Ma, P., Houk, K.N., Shabat, D.
Boosting Chemiexcitation of Phenoxy-1, 2-Dioxetanes through 7-Norbornyl and Homocubanyl Spirofusion
JACS Au, 2024, 4, 3558−3566

158.
David, M., Leirikh, T., Shelef, O., Gutkin, S., Kopp, T., Zhou, Q., Ma, P., Fridman, M., Houk, K.N., Shabat. D.
Chemiexcitation Acceleration of 1, 2-Dioxetanes via a Spiro-Fused Inductive Electron-Withdrawing Motifs
Angew. Chem., 2024, e202410057

157.
Liu, P., Tseng, YL., Ge, L., Zeng, T., Shabat, D., Robb, M.J.
Mechanically Triggered Bright Chemiluminescence from Polymers by Exploiting a Synergy between Masked 2-Furylcarbinol Mechanophores and 1, 2-Dioxetane Chemiluminophores
J. Am. Chem. Soc., 2024, 146, 22151−22156

156.
Tannous, R., Shelef, O., Kopp, T., Fridman, M., Shabat, D.
Hyper-Responsive Chemiluminescent Probe Reveals a Distinct PYRase Activity in Pseudomonas aeruginosa
Bioconjug. Chem., 2024, 35, 472-479

155.
Redy Keisar, O., Pevzner, A., Fridkin, G., Shelef, O., Shabat, D., Ashkenazi, N.
Highly sensitive chemiluminescence sensors for the detection and differentiation of chemical warfare agents
Anal Methods, 2024, 16, 1736-1740

154.
Liubomirski, Y., Tiram, G., Scomparin, A., Gnaim, S., Das, S., Gholap, S., Ge, L., Yeini, E., Shelef, O., Zauberman, A., Berger, N., Kalimi, D., Toister-Achituv, M., Schröter, C., Dickgiesser, S., Tonillo, J., Shan, M., Deutsch, C., Sweeney-Lasch, S., Shabat, D., Satchi-Fainaro, R.
Potent antitumor activity of anti-HER2 antibody-topoisomerase I inhibitor conjugate based on self-immolative dendritic dimeric-linker
J. Control Release, 2024, 367,148-157

153.
Shelef, O., Kopp, T., Tannous, R, Jospe-Kaufman, M., Arutkin, M., Reuveni, S., Shabat, D., Fridman, M.
Enzymatic Activity Profiling Using an Ultra-Sensitive Array of Chemiluminescent Probes for Bacterial Classification and Characterization
J. Am. Chem. Soc., 2024, 146, 5263–5273

152.
Tannous, R., Shelef, O., Gutkin, S., David, M., Leirikh, T., Ge, L., Jaber, J., Zhou, Q., Ma, P., Fridman, M., Spitz, U., Houk, K.N., Shabat, D.
Spirostrain-Accelerated Chemiexcitation of Dioxetanes Yields Unprecedented Detection Sensitivity in Chemiluminescence Bioassays
ACS. Cent. Sci., 2024, 10, 28–42

151.
Gutkin, S., Tannous, R., Jaber, Q., Fridman, M., Shabat, D.
Chemiluminescent Duplex Analysis by Phenoxy-1,2-Dioxetane Luminophores with Color Modulation
Chemical Science, 2023, 14, 6953 - 62

150.
Blau, R., Shelef, O., Shabat, D., Satchi-Fainaro, R.
Chemiluminescent probes in cancer biology
Nat. Rev. Bioeng., 2023, 1, 648–664
149.
Tannous, R., Gutkin, S., Baran, PS., Shabat, D.
Synthesis and Chemiexcitation of a Distinct Chemiluminescent Luminophore based on a Curcumin Scaffold
Isr. J. Chem., 2023, e202300066

148.
David, M., Jaber, Q., Fridman, M., Shabat, D.
Dual Chemiexcitation by a Unique Dioxetane Scaffold Gated by an OR Logic Set of Triggers
Chem. Eur. J., 2023, 29, e202300422

147.
Shelef, O., Gutkin, S., Feder, D., Ben-Bassat, A., Mandelboim, M., Haitin, Y., Ben-Tal, N., Bacharach, E., Shabat, D.
Ultrasensitive Chemiluminescent Neuraminidase Probe for Rapid Screening and Identification of Small-molecules with Antiviral Activity Against Influenza A Virus in Mammalian Cells
Chem. Sci., 2022, 13, 12348–57

146.
Yucknovsky, A., Rich, BB., Gutkin, S., Ramanthrikkovil Variyam, A., Shabat, D., Pokroy, B., and Amdursky, N.
Application of Super Photoacids in Controlling Dynamic Processes: Light-Triggering the Self-Propulsion of Oil Droplets
J. Phys. Chem. B. 2022, 126, 33, 6331–37
145.
Peukert, C., Gholap, S.G., Green, O., Pinkert, L., Heuvel, J.V., Ham, M.V., Shabat, D., and Broenstrup, M.
Enzyme-activated, Chemiluminescent Siderophore-Dioxetane Probes Enable the Selective and Highly Sensitive Detection of Bacterial Pathogens
Angew. Chem. Int. Ed. 2022, 61, e202201423

144.
Gnaim, S., Gholap, S.P., Ge, L., Das, S., Gutkin, S., Green, O., Shelef, O., Hananya, N., Baran, P.S., Shabat, D.
Modular Access to Diverse Chemiluminescent Dioxetane-Luminophores Through Convergent Synthesis
Angew. Chem. Int. Ed. 2022, 61, e202202187

143.
Shelef, O., Gnaim, S., Shabat, D.
Self-Immolative Polymers: An Emerging Class of Degradable Materials with Distinct Disassembly Profiles
J. Am. Chem. Soc. 2021, 143, 21177-88

142.
Shelef, O., Sedgwick, A.C., Pozzi, S., Green, O., Satchi-Fainaro, R., Shabat, D., Sessler, J.L.
Turn-On Chemiluminescence-Based Probes for Monitoring Tyrosinase Activity in Conjunction with Biological Thiols
Chem. Commun., 2021, 57, 11386-89.

141.
Gutkin, S., Gandhesiri, S., Brik, A., Shabat, D
Synthesis and Evaluation of Ubiquitin-Dioxetane Conjugate as a Chemiluminescent Probe for Monitoring Deubiquitinase Activity
Bioconjug. Chem., 2021, 32, 2141-47.

140.
Ponomariov, M., Shabat, D., Green, O
Universal Access to Protease Chemiluminescent Probes through Solid-Phase Synthesis
Bioconjug. Chem., 2021, 32, 2134-40.

139.
Shilo, M., Oved, H., Wertheim, L., Gal, I., Noor, N., Green, O., Baruch, ES., Shabat, D., Shapira, A., Dvir, T
Injectable nanocomposite implants reduce ROS accumulation and improve heart function after infarction
Adv. Sci., 2021, 8, 2102919
138.
Ye, S., Yang, B., Wu, M., Chen, Z., Shen, J., Shabat, D., Yang, D
Recurring Real-Time Monitoring of Inflammations in Living Mice with A Chemiluminescent Probe for Hypochlorous Acid
CCS Chem. 2022, 4, 1871–78

137.
Gholap, S.P., Yao, C., Green, O., Babjak, M., Jakubec, P., Malatinský, T., Ihssen, J., Wick, L., Spitz, U., Shabat, D
Chemiluminescence Detection of Hydrogen Sulfide Release by beta-Lactamase-catalyzed beta-Lactam Biodegradation: Unprecedented Pathway for Monitoring beta-Lactam Antibiotic Bacterial Resistance
Bioconjug. Chem., 2021, 32, 991-1000

136.
Babin, B.M., Fernandez-Cuervo, G., Sheng, J., Green, O., Ordonez, A.A, Turner, M.L., Keller, L.J., Jain, S.K., Shabat, D., Bogyo, M.
Chemiluminescent Protease Probe for Rapid, Sensitive, and Inexpensive Detection of Live Mycobacterium tuberculosis
ACS. Cent. Sci., 2021, 7, 803-14

135.
Scott, JI, Gutkin, S., Green, O., Thompson, EJ., Kitamura, T., Shabat, D., Vendrell, M.
A Functional Chemiluminescent Probe for in vivo Imaging of Natural Killer Cell Activity against Tumours
Angew. Chem. Int. Ed. 2021, 60, 5699-5703

134.
Gutkin, S., Green, O., Raviv, G., Shabat, D., Portnoy, O.
Powerful Chemiluminescence Probe for Rapid Detection of Prostate Specific Antigen Proteolytic Activity: Forensic Identification of Human Semen
Bioconjug. Chem., 2020, 31, 2488-93

133.
Yang, M., Zhang, J., Shabat, D., Fan, J., Peng, X.
Near-Infrared Chemiluminescent Probe for Real-Time Monitoring Singlet Oxygen in Cells and Mice Model
ACS Sensors, 2020, 10, 3158-64.

132.
Ye, S., Hananya, N., Green, O., Chen, H., Qian Zhao, A., Shen, J., Shabat, D., Yang, D.
A Highly Selective and Sensitive Chemiluminescent Probe for Real-Time Monitoring of Hydrogen Peroxide in Cells and Animals
Angew. Chem. Int. Ed. 2020, 59, 14236-30.

131.
Das, S., Ihssen, J., Wick, L., Spitz, U., Shabat, D.
Chemiluminescence Carbapenem-based Molecular Probe for Detection of Carbapenemase Activity in Live Bacteria
Chem. Eur. J., 2020, 26, 3647-52.

130.
Gnaim, S., Shabat, D.
Activity-Based Optical Sensing Enabled by Self-Immolative Scaffolds: Monitoring of Release Events by Fluorescence or Chemiluminescence Output
Acc. Chem. Res., 2019, 52, 2806-17.

129.
Hananya, N., Press, O., Das, A., Scomparin, A., Satchi-Fainaro, R., Sagi, I., Shabat, D.
Persistent Chemiluminescent Glow of Phenoxy-Dioxetane Luminophore Enables Unique CRET-Based Detection of Proteases
Chem. Eur. J., 2019, 25, 14679-87

128.
Miranda-Apodaca, J., Hananya, N., Velázquez-Campoy, A., Shabat, D., Arellano JB.
Emissive Enhancement of the Singlet Oxygen Chemiluminescence Probe after Binding to Bovine Serum Albumin
Molecules, 2019, 24, 2422.
127.
Hananya, N., Shabat, D
Recent Advances and Challenges in Luminescent Imaging: Bright Outlook for Chemiluminescence of Dioxetanes in Water
ACS Central Sci., 2019, 5, 949-59.

126.
Roth-Konforti, M., Green, O., Hupfeld, M., Fieseler, L., Heinrich, N., Ihssen, J., Vorberg, R., Wick, L., Spitz, U., Shabat, D.
Ultrasensitive Detection of Salmonella and Listeria Monocytogenes by Small-Molecule Chemiluminescence Probes
Angew. Chem. Int. Ed. 2019, 58, 10361-67.

125.
Gnaim, S., Shabat, D.
Chemiluminescence Molecular Probe with a Linear Chain Reaction Amplification Mechanism
Org. Biomol. Chem., 2019,17, 1389-94.

124.
Gnaim, S., Scomparin, A., Eldar-Boock, A., Bauer, CR., Satchi-Fainaro, R.,Shabat, D.
Light Emission Enhancement by Supramolecular Complexation of Chemiluminescence Probes Designed for Bioimaging
Chem. Sci., 2019, 10, 2945 - 2955

123.
Son, S., Won, M., Green, O., Hananya, N., Sharma, A., Jeon, Y., Kwak, JH., Sessler, J. L., Shabat, D., Kim, JS.
Chemiluminescent Probe for the In Vitro and In Vivo Imaging of Cancers Over-expressing NQO1
Angew. Chem. Int. Ed. 2019, 58, 1739-94

122.
Hananya, N., Reid, J.P., Green, O., Sigman, M.S., Shabat, D.
Rapid Chemiexcitation of Phenoxy-Dioxetane Luminophores Yields Ultrasensitive Chemiluminescence Assays
Chem. Sci., 2019, 10, 1380-1385

121.
Edri, R., Gal I, Noor, N., Harel, T., Fleischer, S., Adadi, N., Green, O., Shabat, D., Heller, L., Shapira, A,. Gat-Viks, I., Peer, D., Dvir, T.
Personalized Hydrogels for Engineering Diverse Fully Autologous Tissue Implants
Adv. Mater., 2019, e1803895.
120.
Roth-Konforti, M.E., Comune, M., Halperin-Sternfeld, M., Grigoriants, I., Shabat, D., Adler-Abramovich, L.
UV Light-Responsive Peptide-Based Supramolecular Hydrogel for Controlled Drug Delivery
MacroMol Rapid Commun., 2018, 39, 1800588.

119.
Gajst O, Green O, Pinto da Silva L, Esteves da Silva JCG, Shabat D, Huppert D.
Excited-State Proton Transfer to H2O in Mixtures of CH3CN-H2O of a Superphotoacid, Chlorobenzoate Phenol Cyanine Picolinium (CBCyP).
J. Phys. Chem. A. 2018, 122, 8126-8135.
118.
Gnaim, S., Scomparin, A., Das, S., Blau, R., Satchi-Fainaro, R., Shabat, D.
Real-Time Monitoring of Prodrug Activation by Direct-Mode of Chemiluminescence
Angew. Chem. Int. Ed. 2018, 57, 9033-9037

117.
Bruemmer, K.V., Green, O., Su, T. A., Shabat, D., Chang, C. J.
Chemiluminescent Probes for Activity-Based Sensing of Formaldehyde Released from Folate Degradation in Living Mice
Angew. Chem. Int. Ed. 2018, 57, 7508-7512

116.
Blau, R., Epshtein, Y., Tiram, G., Pisarevsky, E., Israeli, S., Yeini,. E., Krivitsky, A., Eldar-Boock, A., Ben-Shushan, D., Green, O., Ben-Nun, Y., Merquiol, E., Schwartz, H., Blum, G., Erez, N., Grossman, R., Ram, Z., Shabat, D., Satchi-Fainaro, R.
Image-Guided Surgery Using Near-Infrared Turn-ON Fluorescent Nanoprobes for Precise Detection of Tumor Margins
Theranostics, 2018, 8, 3437-3460.
115.
Sun, X., Shabat, D., Phillips, S.T., Anslyn, E.V.
Self‐propagating amplification reactions for molecular detection and signal amplification: Advantages, pitfalls, and challenges
J. Phys. Org. Chem. 2018; 31:e3827
114.
Gnaim, S., Shabat, D.
Chemiluminescence Molecular Probe with Intrinsic Auto-Inductive Amplification: Incorporation of Chemiexcitation in a Quinone-Methide Elimination
Chem. Commun., 2018, 54, 2655-58

113.
da Silva, LP., Ori Green, Gajst, O., Simkovitch, R., Shabat, D., Esteves da Silva, J.C.G., Huppert, D.
Excited-State Proton Transfer of Phenol Cyanine Picolinium Photoacid
ACS OMEGA, 2018, 3, 2058-73
112.
Eilon-Shaffer, T., Roth-Konforti, M., Eldar-Boock, A., Satchi-Fainaro, R., Shabat, D.
Ortho-Chlorination of phenoxy 1,2-dioxetane yields superior chemiluminescence probes for in vitro and in vivo imaging
Org. Biomol. Chem., 2018, 16, 1708-12

111.
Gnaim, S., Green, O., Shabat, D.
The Emergence of Aqueous Chemiluminescence: New Promising Class of Phenoxy 1, 2-Dioxetane Luminophores
Chem. Commun., 2018, 54, 2073-85

110.
Gajst, O., Green, O., Simkovitch, R., Shabat, D., Huppert, D.
The photoacidity of phenol chloro benzoate cyanine picolinium salt photoacid in alkanols
J. Photochem. Photobiol. A: Chem. 2018, 353, 546–556.
109.
Roth-Konforti, M., Bauer, C., Shabat, D.
Unprecedented Sensitivity in a Probe for Monitoring Cathepsin B: Chemiluminescence Microscopy Cell-Imaging of a Natively Expressed Enzyme
Angew. Chem. Int. Ed. 2017, 129, 15839-44.

108.
Hananya, N., Shabat, D.
A Glowing Trajectory between Bio- and Chemi-Luminescence: From Luciferin-based Probes to Triggerable Dioxetanes
Angew. Chem. Int. Ed. 2017, 56, 16454-63

107.
Green, O., Gnaim, S., Blau R, Eldar-Boock, A., Satchi-Fainaro, R., Shabat D.
Near-Infrared Dioxetane Luminophores with Direct Chemiluminescence Emission Mode
J. Am. Chem. Soc., 2017, 139, 13242-48.

106.
Green, O., Gajst, O., Simkovitch, R., Shabat, D., Huppert, D.
Chloro Benzoate Cyanine Picolinium Photoacid Excited-State Proton Transfer to Water
J. Photochem. Photobiol. A: Chem. 2017, 349, 230–237.
105.
Hananya N, Green O, Blau R, Satchi-Fainaro R., Shabat D.
A Highly-Efficient Chemiluminescence Probe for Detection of Singlet Oxygen in Living Cells
Angew. Chem. Int. Ed. 2017, 56, 11793-96.

104.
Gnaim, S., Shabat, D.
Self-Immolative Chemiluminescence Polymers: Innate Assimilation of Chemiexcitation in a Domino-Like Depolymerization
J. Am. Chem. Soc., 2017, 139, 10002-08

103.
Gopinath, P., Mahammed, A., Eilon-Shaffer, T., Nawatha, M., Ohayon, S., Shabat, D., Gross, Z., Brik, A.
Switching Futile para‐Quinone to Efficient ROS Generator: Ubiquitin Specific Protease‐2 Inhibition, Electrocatalysis and Quantification
ChemBioChem, 2017, 18, 1683-87.
102.
Green, O., Gajst, O., Simkovitch, R., Shabat, D., Huppert, D.
New Phenol Benzoate Cyanine Picolinium Salt Photoacid Excited-State Proton Transfer
J. Phys. Chem. A. 2017, 16, 3079-87.
101.
Green, O., Eilon, T., Hananya, N., Gutkin, S., Bauer, CR., Shabat, D.
Opening a Gateway for Chemiluminescence Cell Imaging: Distinctive Methodology for Design of Bright Chemiluminescent Dioxetane Probes
ACS Central Sci., 2017, 4, 349-58.

100.
Hananya, N., Eldar-Boock, A., Bauer, CR., Satchi-Fainaro, R., Shabat, D.
Remarkable Enhancement of Chemiluminescent Signal by Dioxetane-Fluorophore Conjugates: Turn-ON Chemiluminescence Probes with Color Modulation for Sensing and Imaging
J. Am. Chem. Soc., 2016, 138, 13438-46.

99.
Shahal, T., Green, O., Hananel, U., Michaeli, Y., Shabat, D., Ebenstein, Y.
Simple and cost-effective fluorescent labeling of 5-hydroxymethylcytosine
Methods and Applications in Fluorescence, 2016, 4, 044003.
98.
Jeffet, J., Kobo, A., Su, T., Grunwald, A., Green, O., Nilsson, AN., Eisenberg, E., Ambjornsson, T., Westerlund, F., Weinhold, E., Shabat, D., Purohit, PK., Ebenstein, Y.
Super-Resolution Genome Mapping in Silicon Nanochannels
ACS Nano, 2016, 10, 9823-30.
97.
Gnaim, S., Scomparin, A., Li, X., Baran, P.S., Rader, C., Satchi-Fainaro, R., Shabat, D.
Tagging the Untaggable: A Difluoroalkyl-Sulfinate Ketone-Based Reagent for Direct C-H Functionalization of Bioactive Heteroarenes
Bioconjugate Chem. 2016, 27, 1965-71.

96.
Green, O., Simkovitch, R., Pinto da Silva, L., Esteves da Silva, JC, Shabat, D., Huppert, D.
Excited-State Proton Transfer and Formation of the Excited Tautomer of 3-Hydroxypyridine-Dipicolinium Cyanine Dye
J. Phys. Chem. A. 2016, 120, 6184-99.
95.
Kisin-Finfer, E., Simkovitch, R., Shabat, D., Huppert, D.
Dormant acceptor activation of 10-hydroxybenzoquinline derivatives by excited-state intramolecular proton transfer
J. Photochem. Photobiol. A: Chem., 2016, 326, 89-99
94.
Herbst, E., Shabat, D.
FRET-based cyanine probes for monitoring ligation reactions and their applications to mechanistic studies and catalyst screening
Org. Biomol. Chem., 2016, 12, 3715-28

93.
Shaulov-Rotem, Y., Merquiol, E., Weiss-Sadan, T., Ofra Moshel , Salpeter, S., Shabat, D., Kaschani, F., Kaiser, M., Blum, G.
A novel quenched fluorescent activity-based probe reveals caspase-3 activity in the endoplasmic reticulum during apoptosis
Chem. Sci., 2016, 7, 1322-37.
92.
Roth, M. E., Green O., Gnaim S., Shabat D.
Dendritic, Oligomeric, and Polymeric Self-Immolative Molecular Amplification.
Chem. Rev., 2016, 116, 1309-52

91.
Kisin-Finfer E., Redy-Keisar O., Roth M., Ben-Eliyahu R., Shabat D.
Molecular Insight into Long-Wavelength Fluorogenic Dye Design: Hydrogen Bond Induces Activation of a Dormant Acceptor
Chem. Eur. J., 2015, 21, 18566-70

90.
Redy-Keisar, O.,Ferber, S., Satchi-Fainaro, R., Shabat, D.
NIR fluorogenic dye as a modular platform for prodrug assembly:Real-time in vivomonitoring of drug release
ChemMedChem, 2015, 10, 999-1007.

89.
Redy-Keisar, O., Huth, K.,Vogel, E.,Lepenies, B., Seeberger, P.H., Haag., R., Shabat, D.
Enhancement of Fluorescent Properties of Near-Infrared Dyes using Clickable Oligoglycerol Dendrons
Org. Biomol. Chem., 2015, 13, 4727-32.

88.
Gnaim, S., Shabat, D.
Quinone-Methide Species, A Gateway to Functional Molecular Systems: From Self-Immolative Dendrimers to Long-Wavelength Fluorescent Dyes
Acc. Chem. Res., 2014, 47, 2970-2984

87.
Shahal-Koren, T., Gilat, N., Michaeli, Y., Redy-Keisar, O., Shabat, D., Ebenstein, Y.
Spectroscopic quantification of global 5'hydroxymethylcytosine in genomic DNA
Anal. Chem., 2014, 86, 8231-7.
86.
Simkovitch, R., Akulov K., Shomer, S., Roth M.E., Shabat, D., Schwartz, T., Huppert, D.
Comprehensive Study of Ultrafast ESPT in Water and D2O Provides the Missing RO-⋯H+ Ion-Pair Fingerprint
J. Phy. Chem. A, 2014, 118, 4425-43.
85.
Kisin-Finfer., Ferber, S., Blau, R., Satchi-Fainaro, R., Shabat, D.
Synthesis and Evaluation of New NIR-FluorescentProbes for Cathepsin B: ICT vs. FRET as a Turn-ON Mode-of-Action
Bioorg. Med. Chem. Lett., 2014, 24, 2453-58.

84.
Ferber, S., Baabur-Cohen, H., Blau, B., Epshtein, Y., Kisin-Finfer, E., Redy, O., Shabat, D., Satchi-Fainaro, R.
Polymeric nanotheranostics for real-time non-invasive optical imaging of breast cancer progression and drug release
Cancer Lett., 2014, 352, 81-89
83.
Simkovitch, R., Shomer, S., Gepshtein, R., Shabat, D., Huppert, D.
Excited-State Proton Transfer from Quinone-Cyanine 9 to Protic Polar-Solvent Mixtures
J. Phy. Chem. A, 2014, 118, 1832-40.
82.
Mizrahy, S., Goldsmith, M., Leviatan-Ben-Arye, S., Kisin-Finfer, E., Redy, O., Srinivasan, S., Godlin, B., Shabat, D., Godin, B., Peer, D.
Tumor targeting profiling of hyaluronan-coated lipid based- nanoparticles
Nanoscale, 2014, 6, 3742-52
81.
Cohen K, Emmanuel R, Kisin-Finfer E, Shabat D, Peer D.
Modulation of Drug Resistance in Ovarian Adenocarcinoma using Chemotherapy Entrapped in Hyaluronan-Grafted Nanoparticle Clusters
ACS Nano, 2014, 8, 2183-2195.
80.
Redy, O., Kisin-Finfer, E., Ferber, S., Satchi-Fainaro, R., Shabat, D.
Synthesis and Use of QCy7-derived Modular Probes for Detection and Imaging of Biologically Relevant Analytes
Nature Protocols, 2014, 9, 27-36.

79.
Simkovitch, R., Shomer, S., Gepshtein, R., Roth, M. E., Shabat, D., Huppert, D.
Comparison of the Rate of Excited-State Proton Transfer from Photoacids to Alcohols and Water
J. Photochem. Photobiol. A: Chemistry, 2014, 277, 90-101.
78.
Sella, E., Shabat, D.
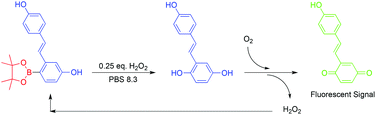
Hydroquinone-quinone oxidation by molecular oxygen: a simple tool for signal amplification through auto-generation of hydrogen peroxide
Org. Biomol. Chem., 2013, 11, 5074-8.
77.
Simkovitch, R., Karton-Lifshin, N., Shomer, S., Shabat, D., Huppert, D.
Ultrafast Excited-State Proton Transfer to the Solvent Occurs on a Hundred-Femtosecond Time-Scale
J. Phy. Chem. A, 2013, 117, 3405-13.
76.
Simkovitch, R., Shomer, S., Gepshtein, R., Shabat, D., Huppert. D.
Temperature Dependence of the Excited-State Proton-Transfer Reaction of QCy7
J. Phy. Chem. A, 2013, 3925-34.
75.
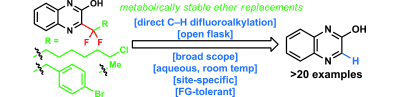
Zhou, Q., Ruffoni, A., Rianatassio, R., Fujiwara, Y., Sella, E., Shabat, D. Baran, PS.
Direct Synthesis of Fluorinated Heteroarylether Bioisosteres
Angew. Chem. Int. Ed. Engl., 2013, 52, 3949-52.
74.
Kisin-Finfer., E., Shabat, D.
New Repertoire of "Donor-Two-Acceptor" NIR Fluorogenic Dyes
Bioorg. Med. Chem. Lett., 2013, 21, 3602-8.

73.
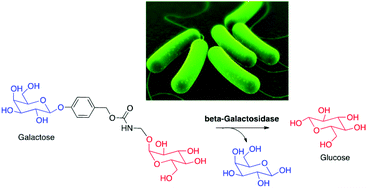
Karton-Lifshin, N., Vogel, U., Sella, E., Seeberger, P.H., Shabat, D., Lepenies, B.
Enzyme-mediated nutrient release: glucose-precursor activation by β-galactosidase to induce bacterial growth
Org. Biomol. Chem., 2013, 11, 2903-10.
72.
Simkovitch, R., Kisin-Finfer, E., Shomer, S., Erez, Y., Gepshtein, R., Shabat D., Huppert, D.
Ultrafast Excited-State Proton Transfer from Hydroxycoumarin-Dipicolinium Cyanine Dyes
J. Photochem. Photobiol. A: Chem., 2013, 254, 45-53
71.
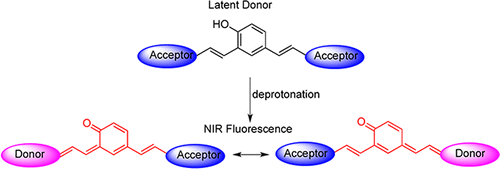
Karton-Lifshin N,Albertazzi L,Bendikov M,Baran PS,Shabat D.
Donor-two-acceptor" dye design: a distinct gateway to NIR fluorescence
J Am Chem Soc., 2012, 134, 20412-20.
70.
Presiado I,Karton-Lifshin N,Erez Y,Gepshtein R,Shabat D,Huppert D.
Ultrafast proton transfer of three novel quinone cyanine photoacids
J Phys Chem A., 2012, 116, 7353-63.
69.
Redy, O., Shabat, D.
Modular Theranostic Prodrug based on a FRET-Activated Self-Immolative Linker
J. Control. Release , 2012, 164, 276-82.

68.
Huppert, D., Shabat, D., Presiado, I., Karton-Lifshin, N., Erez, Y., Gepshtein, R.
Ultrafast Excited-State Intermolecular Proton Transfer of Cyanine Fluorochrome Dyes
J. Phy. Chem. A, 2012, 116, 5-92.
67.
Redy, O., Kisin-Finfer, E., Sella, E., Shabat, D.
A Simple FRET-Based Modular Design for Diagnostic Probes
Org. Biomol. Chem., 2012, 10, 710-5.

66.
Karton-Lifshin, N., Shabat, D.
Exponential Diagnostic Signal Amplification via Dendritic Chain Reaction: The Dendritic Effect of a Self-Immolative Amplifier Component
New, J. Chem., 2012, 36, 386-93.
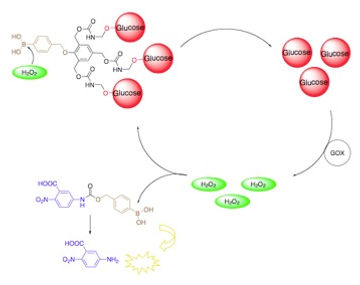
65.
Perry-Feigenbaum, R., Sella, E., Shabat, D.
AutoInductive Exponential Signal Amplification: A Diagnostic Probe for Detection of Fluoride
Chem. Eur. J., 2011, 17, 12123-8.

64.
Karton-Lifshin, N., Segal, E., Omer, L., Portnoy, M., Satchi-Fainaro, R., Shabat, D.
A Unique Paradigm for a Turn-ON Near-Infrared Cyanine-Based Probe: Non-Invasive Intravital Optical Imaging of Hydrogen Peroxide
J. Am. Chem. Soc., 2011, 133, 10960-5.

63.
Sella, E., Weinstain, R., Erez, R., Burns, Z. N., Baran, S. P., Shabat, D.
Sulfhydryl-Based Dendritic Chain Reaction
Chem. Commun., 2010, 21, 6575-7.

62.
Avital-Shmilovici, M., Shabat, D.
Dendritic Chain Reaction: Responsive Release of Hydrogen Peroxide upon Generation and Enzymatic Oxidation of Methanol
Bioorg. Med. Chem., 2010, 18, 3643-7.

61.
Sella, E., Lubelski, A., Klafter, J., Shabat, D.
Two-Component Dendritic Chain Reactions: Experiment and Theory
J. Am. Chem. Soc., 2010, 132, 3945-52.
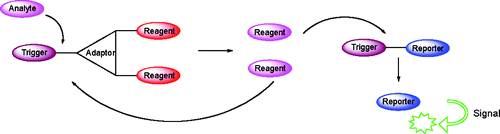
60.
Avital-Shmilovici, M., Shabat, D.
Self-Immolative Dendrimers: A Distinctive Approach to Molecular Amplification
Soft Matter, 2010, 6, 1073-1080.
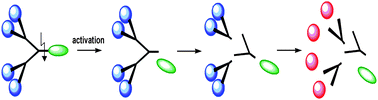
59.
Weinstain, R., Segal, E., Satchi-Fainaro, R., Shabat, D.
Real-Time Monitoring of Drug Release
Chem. Commun., 2010, 46, 553-5.
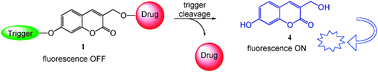
58.
Perry-Feigenbaum, R., Baran, S. P., Shabat, D.
The Pyridinone-Methide Elimination
Org. Biomol. Chem., 2009, 7, 4825-4828.
57.
Weinstein, R., Baran, S. P., Shabat, D.
Activity-Linked Labelling of Enzymes by Self-Immolative Polymers
Bioconjug. Chem., 2009, 20, 1783-1791.

56.
Sella, E., Shabat, D.
Dendritic Chain Reaction
J. Am. Chem. Soc., 2009, 131, 9934-6.
55.
Avital-Shmilovici, M., Shabat, D.
Enzymatic Activation of Hydrophobic Self-Immolative Dendrimers: The Effect of Reporters with Ionizable Functional Groups
Bioorg. Med. Chem. Lett., 2009, 19, 3959-62.

54.
Erez, R., Segal, E., Miller, K., Satchi-Fainaro, R., Shabat, D.
Enhanced Cytotoxicity of a Polymer-Drug Conjugate with Triple Payload of Paclitaxel
Bioorg. Med. Chem., 2009, 17, 4327-35.

53.
Stern, L., Perry, R., Ofek, P., R., Many, A., Shabat, D., Satchi-Fainaro, R.
A novel antitumor prodrug designed to be cleaved by the endoprotease legumain
Bioconjug. Chem., 2009, 20, 500-10.
52.
Miller, K., Erez, R., Segal, E., Shabat, D., Satchi-Fainaro, R.
Targeting Bone Metastases with a Bispecific Anticancer and Antiangiogenic Polymer–Alendronate–Taxane Conjugate
Angew. Chem. Int. Ed. Engl., 2009, 48, 2949-54.

51.
Sella, E., Shabat, D.
Self-Immolative Dendritic Probe for Direct Detection of Triacetone Triperoxide
Chem. Commun., 2008, 44, 5701-3.
50.
Erez, R., Shabat, D.
The Azaquinone-Methide Rearrangement: Comparison Study of 1, 6- and 1, 4-Eliminations under Physiological Conditions
Org. Biomol. Chem., 2008, 6, 2669-72.

49.
Weinstain, R., Sagi, A., Karton, N., Shabat, D.
Self-Immolative Comb-Polymers: Multiple-Release of Side-Reporters by a Single Stimulus Event
Chem. Eur. J., 2008, 14, 6857-61.

48.
Sagi, A., Weinstain, R., Karton, N., Shabat, D.
Self-Immolative Polymers
J. Am. Chem. Soc., 2008, 130, 5434-5.

47.
Erez, R., Ebner, S., Attali, B., Shabat, D.
Chemotherapeutic bone-targeted bisphosphonate prodrugs with hydrolytic mode of activation
Bioorg. Med. Chem. Lett., 2008, 18, 816-20.

46.
Peretz, A., Degani-Katzav, M., Talmon, M., Danieli, E., Gopin, A., Malka, E., Nachman, R., Raz, A., Shabat, D., Attali, B.
A tale of switched functions: from cyclooxygenase inhibition to M-channel modulation in novel diphenylamine derivatives
PLoS ONE, 2007, 2, e1332.
45.
Danieli, E., Shabat, D.
Molecular Probe for Enzymatic Activity with Dual Output
Bioorg. Med. Chem. Lett., 2007, 15, 7318-24.

44.
Sagi, A., Segal, E., Satchi-Fainaro, R., Shabat, D.
Remarkable Drug-Release Enhancement with an Elimination-based AB3 Self-Immolative Dendritic Amplifier
Bioorg. Med. Chem., 2007, 15, 3720-7.

43.
Abramovich, L.A., Perry, R., Sagi, A., Gazit, E., Shabat, D.
Controlled Assembly of Peptide Nanotubes Triggered by Enzymatic Activation of Self-Immolative Dendrimers
ChemBioChem, 2007, 8, 859-62.

42.
Shamis, M., Shabat, D.
Single-Triggered AB6 Self-Immolative Dendritic Amplifier
Chem. Eur. J., 2007, 13, 4253-8.

41.
Perry, R., Amir, RJ., Shabat, D.
Substituent-Dependent Disassembly of Self-Immolative Dendrimers
New J. Chem., 2007, 31, 1307-12.
40.
Shamis, M., Barbas, C.F. III., Shabat, D.
A New Visual Screening Assay for Catalytic Antibodies with retro-Aldol retro-Michael Activity
Bioorg. Med. Chem. Lett., 2007, 17, 1172-5.

39.
Peretz, A., Degani, N., Uziyel, Y., Gopin, A., Shabat, D., Attali, B.
Pre- and Post-Synaptic Activation of M-Channels by a Novel Opener Dampens Neuronal Firing and Transmitter Release
J. Neurophysiol., 2007, 97, 283-95.
38.
Amir, R.J., Danieli, E., Gopin, A., Shabat, D.
Receiver-Amplifier, Self-Immolative Dendritic Device
Chem. Eur. J., 2007, 13, 812-821.

37.
Gopin, A., Ebner, S., Attali, B., Shabat, D.
Enzymatic Activation of Second-Generation Dendritic Prodrugs: Conjugation of Self-Immolative Dendrimers with Polyethylene Glycol via Click Chemistry
Bioconjugate Chem., 2006, 17(6), 1432-1440.

36.
Shabat, D.
Self-Immolative Molecular Dendritic Systems
Bulletin of Israel Chemical Society, 2006, 22, 11-18.
35.
Yacoby, I., Shamis, M., Shabat, D., Benhar, I.
Targeting anti bacterial agents by drug-carrying filamentous bacteriophages
Antimicrob. Agents Chemother., 2006, 50(6), 2087-97.
34.
Shabat, D.
Self-Immolative Dendrimers as Novel Drug Delivery Platforms
J. Poly. Sci. Part A, 2006, 44(5), 1569-1578.

33.
Sagi, A., Rishpon, J., Shabat. D.
Amperometric Assay for Aldolase Activity: Antibody-Catalyzed Ferroceneamine Formation
Anal. Chem., 2006, 78(5), 1459-1461.

32.
Amir, R.J., Shabat, D.
Domino Dendrimers
Adv. Polym. Sci., 2006, 192: 59-93.

31.
Weinstain R, Lerner, R. A., Barbas C. F. III., Shabat, D.
Antibody-Catalyzed Asymmetric Intramolecular Michael Addition of Aldehydes and Ketones to Yield the disfavored Cis-Product
J. Am. Chem. Soc., 2005, 127, 13104-5.
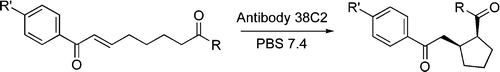
30.
Amir R. J., Popkov, M., Lerner, R. A., Barbas C. F. III., Shabat, D.
Prodrug Activation Gated by a Molecular OR Logic Trigger
Angew. Chem., 2005, 44, 4378-81.

29.
Peretz, A., Degani, N., Uziyel, Y., Shabat, D., Attali, B.
Meclofenamic Acid and Diclofenac, Novel Templates of KCNQ2/Q3 Potassium Channel Openers, Depress Cortical Neuron Activity and Exhibit Anticonvulsant Properties
Molecular Pharmacology, 2005, 67, 1053-66.
28.
Flomenbom, O., Amir, R.J., Shabat, D., Klafter, J.
Some New Aspects of Dendrimer Applications
J. of Luminescence, 2005, 111, 315-25.
27.
Haba, K., Popkov, M., Shamis, M., Lerner, R. A., Barbas C. F. III., Shabat, D.
Single-Triggered Trimeric Prodrugs
Angew. Chem., 2005, 44, 716-20.

26.
Amir, R.J., Shabat, D.
Self-Immolative Dendrimer Biodegradability by Multi-Enzymatic Triggering
Chem. Commun., 2004, 21,1614-5.
25.
Shabat, D., Amir, R.J., Gopin, A., Pessah, N., Shamis, M.
A Chemical Adaptor System Designed To Link a Tumor‐Targeting Device with a Prodrug and an Enzymatic Trigger
Chem. Eur. J., 2004, 10, 2626-34.

24.
Gopin, A., Rader, C., and Shabat, D.
New Chemical Adaptor Unit Designed to Release a Drug from a Tumor Targeting Device by Enzymatic Triggering
Bioorg. Med. Chem., 2004, 12, 1853-8.

23.
Pessah, N., Reznik, M., Shamis, M., Yantiri, F., Xin, H., Bowdish, K., Shomron, N., Ast, G., Shabat, D.
Bioactivation of Carbamate-Based 20(S)-Camptothecin Prodrugs
Bioorg. Med. Chem., 2004, 12, 1859-66.

22.
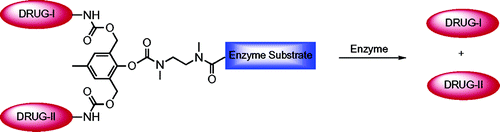
Shamis, M., Lode, H.N., Shabat, D.
Bioactivation of Self-Immolative Dendritic Prodrugs by Catalytic Antibody 38C2
J. Am. Chem. Soc., 2004, 126, 1726-31.
21.
Amir, R.J., Pessah, N., Shamis, M., and Shabat, D.
Self-Immolative Dendrimers
Angew Chem Int Ed Engl., 2003, 42, 4494-4499.

20.
Rader, C., Turner, J.M., Heine, A., Shabat, D., Sinha, S.C., Wilson, I.A., Lerner, R.A., and Barbas, C.F.
A humanized aldolase antibody for selective chemotherapy and adaptor immunotherapy
J. Mol. Biol., 2003, 332, 889-899.
19.
Jikai, J., Shamis, M., Huebener, N., Schroeder, U., Wrasidlo, W., Wenkel, J., Lange, B., Gaedicke, G., Shabat, D., and Lode, H.N.
Neuroblastoma directed therapy by a rational prodrug design of etoposide as a substrate for tyrosine hydroxylase
Cancer Lett., 2003, 197, 219-224.
18.
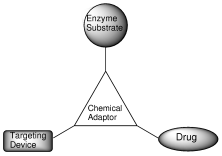
Gopin, A., Pessah, N., Shamis, M., Rader, C., and Shabat, D.
A chemical adaptor system designed to link a tumor-targeting device with a prodrug and an enzymatic trigger
Angew. Chem. Int. Ed. Engl., 2003, 42, 327-332.
17.
Schroeder, U., Bernt, K.M., Lange, B., Wenkel, J., Jikai, J., Shabat, D., Amir, R., Huebener, N., Niethammer, A.G., Hagemeier, C., Wiebusch, L., Gaedicke, G., Wrasidlo, W., Reisfeld, R.A., and Lode, H.N.
Hydrolytically activated etoposide prodrugs inhibit MDR-1 function and eradicate established MDR-1 multidrug-resistant T-cell leukemia
Blood, 2003, 102, 246-253.
16.
Wrasidlo, W., Schroder, U., Bernt, K., Hubener, N., Shabat, D., Gaedicke, G., and Lode, H.
Synthesis, hydrolytic activation and cytotoxicity of etoposide prodrugs
Bioorg. Med. Chem. Lett., 2002, 12, 557-560.
15.
Satchi-Fainaro, R., Wrasidlo, W., Lode, H.N., and Shabat, D.
Synthesis and characterization of a catalytic antibody-HPMA copolymer-Conjugate as a tool for tumor selective prodrug activation
Bioorg. Med. Chem., 2002, 10, 3023-3029.

14.
Shabat, D., Lode, H.N., Pertl, U., Reisfeld, R.A., Rader, C., Lerner, R.A., and Barbas, C.F., 3rd.
In vivo activity in a catalytic antibody-prodrug system: Antibody catalyzed etoposide prodrug activation for selective chemotherapy
Proc. Natl. Acad. Sc.i U S A, 2001, 98, 7528-7533.
13.
Shabat, D., Rader, C., List, B., Lerner, R.A., and Barbas, C.F., 3rd.
Multiple event activation of a generic prodrug trigger by antibody catalysis
Proc Natl Acad Sci U S A, 1999, 96, 6925-6930.
12.
List, B.; Shabat, D.; Zhong, G.; Turner, J. M.; Li, A.; Bui, T.; Anderson, J.; Lerner, R. A.; Barbas, C. F., III
A Catalytic Enantioselective Route to Hydroxy-Substituted Quaternary Carbon Centers: Resolution of Tertiary Aldols with a Catalytic Antibody
J. Am. Chem. Soc., 1999, Vol. 121, pp 7283-7291.
11.
Shabat, D., List, B., Lerner, R. A. & Barbas, C. F., III.
A short enantioselective synthesis of 1-deoxy-L-xylulose by antibody catalysis
Tetrahedron Lett., 1999, 40, 1437-1440.
10.
Zhong, G.; Shabat, D.; List, B.; Anderson, J.; Sinha, S. C.; Lerner, R. A.; Barbas, C. F., III.
Catalytic enantioselective retro-aldol reactions: kinetic resolution of b-hydroxyketones with aldolase antibodies
Angew. Chem., Int. Ed., 1998, 37, 2481-2484.
09.
List, B.; Shabat, D.; Barbas, C. F., III; Lerner, R. A.
Enantioselective total synthesis of some brevicomins using aldolase antibody 38C2
Chem.--Eur. J., 1998, 4, 881-885.
08.
Hoffmann, T.; Zhong, G.; List, B.; Shabat, D.; Anderson, J.; Gramatikova, S.; Lerner, R. A.; Barbas, C. F., III.
Aldolase Antibodies of Remarkable Scope
J. Am. Chem. Soc. 1998, 120, 2768-2779.
07.
Shulman, A.; Keinan, E.; Shabat, D.; Barbas, C. F., III
Teaching catalytic antibodies to undergraduate students: an organic chemistry lab experiment
J. Chem. Educ., 1999; Vol. 76, pp 977-982.
06.
Shabat, D.; Shulman, H.; Itzhaky, H.; Reymond, J.-L.; Keinan, E.
Enantioselectivity vs. kinetic resolution in antibody catalysis: formation of the (S) product despite preferential binding of the (R) intermediate
Chem. Commun. (Cambridge), 1998, 1759-1760.
05.
Shabat, D.; Grynszpan, F.; Saphier, S.; Turniansky, A.; Avnir, D.; Keinan, E.
An efficient sol-gel reactor for antibody-catalyzed transformations
Chem. Mater., 1997, 9, 2258-2260.
04.
Shabat, D.; Sinha, S. C.; Reymond, J.-L.; Keinan, E.
Catalytic antibodies as probes of evolution: modeling of a primordial glycosidase
Angew. Chem., Int. Ed. Engl., 1996, 35, 2628-2632.
03.
Keinan, E.; Sinha, S. C.; Shabat, D.; Itzhaky, H.; Reymond, J.-L.
Asymmetric organic synthesis with catalytic antibodies
Acta Chem. Scand., 1996, 50, 679-687.
02.
Ghosh, P.; Shabat, D.; Kumar, S.; Sinha, S. C.; Grynszpan, F.; Li, J.; Noodleman, L.; Keinan, E.
Using antibodies to perturb the coordination sphere of a transition metal complex
Nature, 1996, 382, 339-341.
01.
Shabat, D.; Itzhaky, H.; Reymond, J.-L.; Keinan, E.
Antibody catalysis of a reaction otherwise strongly disfavored in water
Nature, 1995, 374, 143-5.

